Stretch marks: In vitro study and assessment of combined treatment with PRP and sodium ascorbate on fibroblasts
Stretch marks (SD, Striae Distensae) evolve in two clinical phases, an initial inflammatory phase in which they are called ‘‘striae rubrae’’ (SR) and a chronic phase in which they are called “striae albae” (SA).
The purpose of this study was to describe and analyze stretch mark-derived fibroblasts (SMF) and the differences between SR- and SA-derived fibroblasts (SRF, SAF), by testing two treatments in vitro (PRP [RegenKit®-BCT] and sodium ascorbate) and their combination on SAF.
It is accepted that SAF are quiescent and produce lower amounts of collagen compared to normal skin-derived fibroblasts.
SAF treated with PRP, sodium ascorbate or their combination showed a resumption of their metabolic activity as indicated by an increase in collagen type I production (ELISA quantification). Concentrated PRP (additional centrifugation with higher spin for 10 min and elimination of half of the platelet poor plasma) showed a non-significant effect on collagen production (p = 0.123).
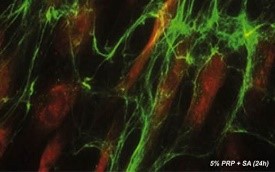
After 24 h of incubation with 1% PRP or 5% PRP both combined with sodium ascorbate, cell viability was increased by 140% and 151% and by 156% and 178% after 48 h, respectively, compared to the control (p < 0.005). Concentrated PRP had a small or even negative effect on cellular viability in all experiments.
This work shows that a biologically mediated improvement of the metabolic activity of fibroblasts from white stretch marks (SAF) is possible in vitro by the addition of PRP and opens the way to future clinical applications.
La Padula, S., Hersant, B., Pizza, C. et al. Striae Distensae: In Vitro Study and Assessment of Combined Treatment With Sodium Ascorbate and Platelet-Rich Plasma on Fibroblasts. Aesth Plast Surg 45, 1282–1293 (2021).
Levoux J, Prola A, Lafuste P, Gervais M, Chevallier N, Koumaiha Z, Kefi K, Braud L, Schmitt A, Yacia A, Schirmann A, Hersant B, Sid-Ahmed M, Ben Larbi S, Komrskova K, Rohlena J, Relaix F, Neuzil J, Rodriguez AM. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021 Feb 2;33(2):283-299.e9. doi: 10.1016/j.cmet.2020.12.006. Epub 2021 Jan 4. Erratum in: Cell Metab. 2021 Mar 2;33(3):688-690.
Bruno BOEZENNEC, MD, Managing Editor
