Our Technologies
The RegenLab® USA technology is based on the use of a thixotropic gel to separate blood components. Thixotropy is a physical property of some gels. The gel acts as a mechanical separator of the blood components. To learn more about the patents protecting this process, please visit www.regenlabusa.com/patents.
Thixotropy is a physical property of some gels. The gel is thick under static conditions. It becomes more fluid when submitted to physical stress (in this case, a centrifugal force of 1500 g) and regains its original consistency when the physical stress stops (Barnes, 1997).
The gel acts as a mechanical separator of the blood components. Two types of thixotropic gels with different specific densities are used in Regen Lab devices. One type is designed to separate platelets only, while the other allows the recovery of platelets with mononuclear cells (lymphocytes and monocytes). Performance studies have shown that the Regen tubes are reliable and produce a consistent separation of platelets and plasma from erythrocytes and leukocytes.
Regen Lab medical devices consist of evacuated pharmaceutical grade borosilicate tubes that contain a pharmaceutical grade anticoagulant solution and a separating polymer gel specifically designed for PRP isolation. The vacuum in the tube allows the preparation of PRP in fully closed circuit. A predetermined amount of blood (8-10 mL) is automatically collected in the tube by connecting it to a blood collection set. Using an evacuated tube for blood collection is not only less traumatic for the blood than using a syringe but is also safer for the operator and the patient.
Blood is mixed with the pre-loaded anticoagulant by gentle inversion of the tube and centrifuged for 5 to 9 minutes depending on the model of tube and the type of separating gel. The centrifugation can be performed using either a small tabletop centrifuge equipped with a 45° fixed angle rotor or in a larger centrifuge with a swing out horizontal rotor.
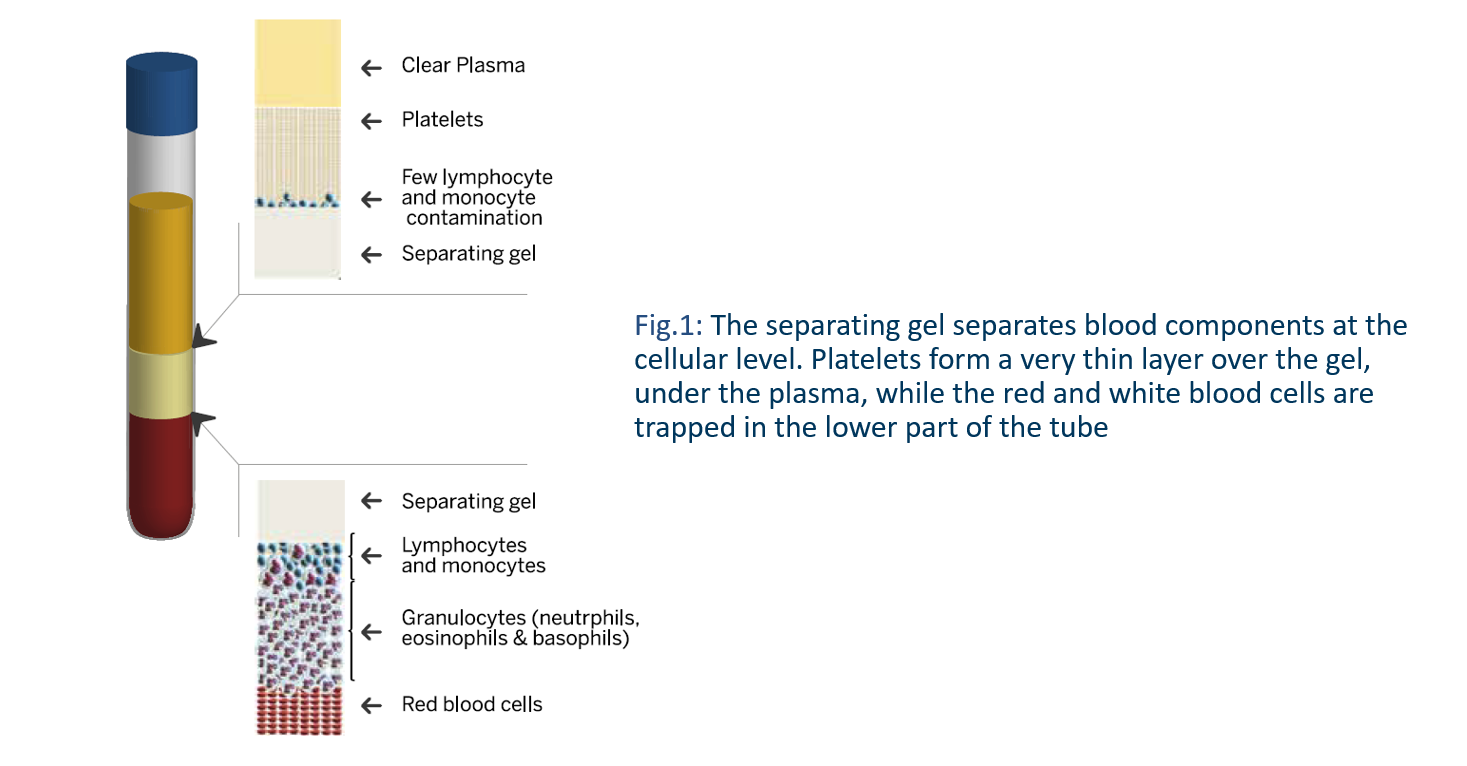
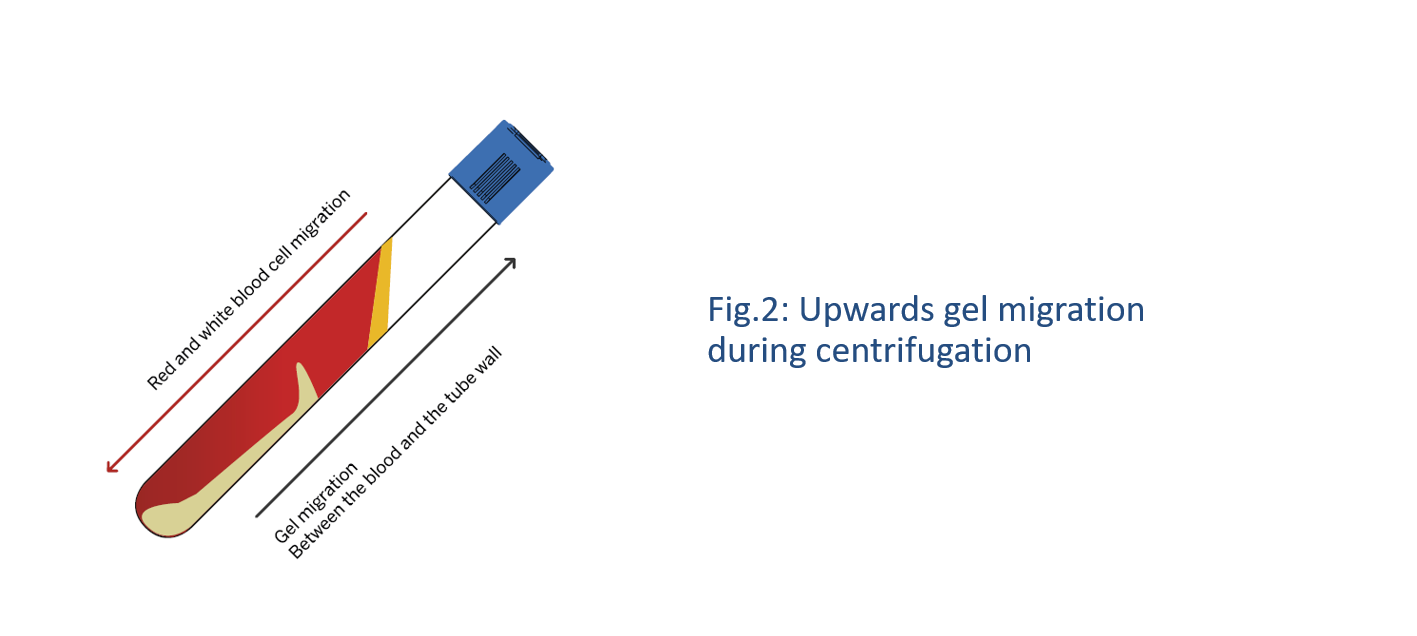
During centrifugation, the gel migrates upwards in the device, while blood cells migrate downwards between the separating gel and the wall of the tube (Fig. 1). The gel is biologically inert. It does not act as a filter but rather as a density gradient medium like Ficoll®, however in a solid form. It intercalates itself precisely between blood components at the cellular level and when it regains its original consistency at the end of the centrifugation, it forms a solid barrier that isolates platelets and plasma from the other blood elements (Fig. 2).
Due to the centrifugal force, all of the platelets are packed on the upper surface of the gel forming a thin sediment. The full volume of plasma is recovered above the separating gel. At the end of centrifugation, the plasma is deprived of cellular elements and thus is transparent platelet-poor plasma (PPP). The platelets are resuspended in the plasma by repeated gentle inversions (at least 20 times) of the tube and the plasma becomes turbid.
The resulting PRP (approx. 5 mL) is then collected using a blood transfer device connected to a syringe. The PRP preparation is now ready for use by the physician. The complete procedure can be performed within 20 minutes. With this technology there areonly two sterile barrier entries (blood in/PRP out). This minimizes the risks of bacterial contamination of the blood sample and blood exposure for the operator.
All Regen Lab kits have undergone biocompatibility and safety testing. The RegenKit® product families have been designed, manufactured, and packaged in line with the relevant harmonized European and other international standards to ensure that they are in accordance with the current international guidelines. Regen Lab kits have a CE certificate and have obtained clearance in almost all countries (USA, Canada, Australia, People’s Republic of China…).
RegenKit®-Wound Gel with FDA Clearance
RegenLab® USA has also developed a wound healing gel that has received FDA Clearance (BK210661). This gel is designed to accelerate the wound healing process by creating an optimal healing environment, promoting tissue regeneration, and protecting the wound from infection. It has undergone rigorous testing to meet the highest safety and efficacy standards, ensuring its suitability for a wide range of wound care applications. This innovation aligns with RegenLab® USA’s commitment to developing cutting-edge, biocompatible medical devices that enhance patient outcomes.
Contact Regenlab® USA
Now is the time to step into the future of medicine.
Join the Generation!
| REGENeration |
Get connected with us to Learn More.
For more information on our products, please contact us by clicking on the button below:
Our Affiliated Locations:
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)
